










On July 4, 2025, XFT officially joined the Shenzhen Pingshan District Medical & Health Data Alliance and was recognized by the local government.
At the same event, the district’s Science & Technology Innovation Bureau released the first batch of the Clinical Specialty Medical Devices Catalog, featuring three of our key products: the XFT‑2003EA and XFT‑2003E Hand Function Rehabilitation Devices, and the XFT‑2003K Biofeedback Electrical Stimulator. This highlights our strength in medical data compliance and innovation, and marks a key step toward enhancing product value through data-driven development.

The Shenzhen Pingshan District Medical & Health Data Alliance was launched jointly by the district’s Science & Technology Innovation Bureau and the Health Commission. It serves as a key platform to break down data barriers across government, industry, academia, research, and clinical practice. By joining, XunFengTong not only gains official recognition but also gains priority access to alliance resources, policies, and projects—injecting data momentum into our core rehabilitation and IVD businesses, and giving us a head start in the “Medical + AI + Data” convergence space.
 The Pingshan District Clinical Specialty Medical Devices Catalog is designed to promote local medical innovation, accelerate the clinical application of new technologies, and support the high-quality. After a rigorous review, three XFT products were selected for their innovation, clinical value, and commercialization potential.
The Pingshan District Clinical Specialty Medical Devices Catalog is designed to promote local medical innovation, accelerate the clinical application of new technologies, and support the high-quality. After a rigorous review, three XFT products were selected for their innovation, clinical value, and commercialization potential.
These products focus on rehab solutions for central nervous system injuries—such as stroke and spinal cord injury—using biofeedback electrical stimulation to provide precise and personalized care.
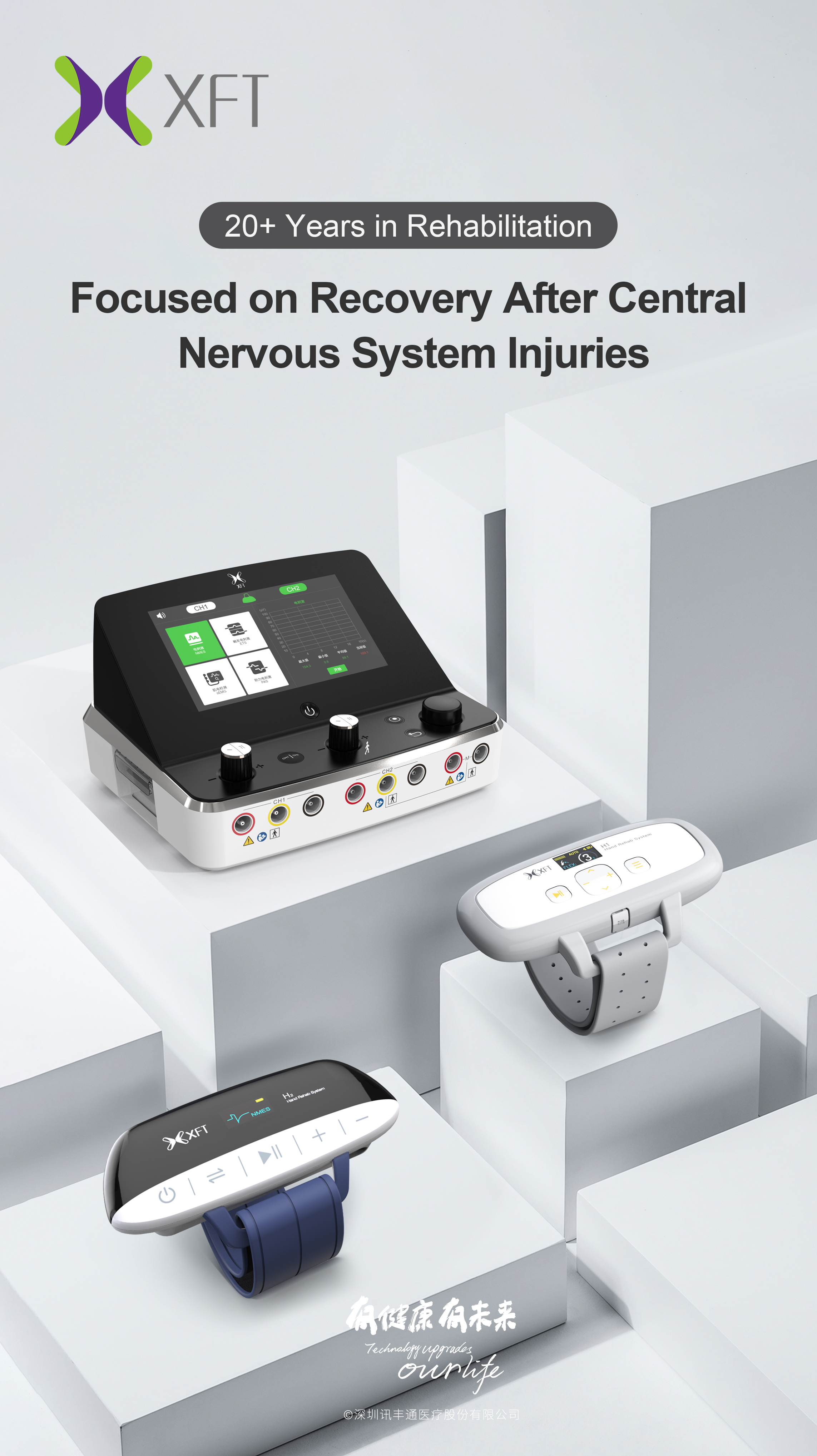 XFT‑2003E / XFT‑2003EA Hand Function Rehabilitation Device: designed for patients with impaired hand function, these wearable devices use functional electrical stimulation to re-activate neuromuscular pathways. Lightweight and suitable for home use, they offer multiple modes covering the entire rehab cycle—helping users regain the ability to “hold” onto life.
XFT‑2003E / XFT‑2003EA Hand Function Rehabilitation Device: designed for patients with impaired hand function, these wearable devices use functional electrical stimulation to re-activate neuromuscular pathways. Lightweight and suitable for home use, they offer multiple modes covering the entire rehab cycle—helping users regain the ability to “hold” onto life.
XFT‑2003K Biofeedback Elect rical Stimulator: this device captures the patient’s residual micro-electromyographic (EMG) signals and triggers synchronized electrical stimulation based on muscle strength, promoting muscle contraction. It aids in restoring autonomous limb movement and prevents muscle atrophy and joint contracture. With five distinct functional modes—from basic EMG detection to mirror therapy—it supports data-driven, personalized rehab plans. An innovative feature is the negative-pressure electrode: adjustable suction ensures firm adhesion, excellent conductivity, and reduced need for consumable replacement—balancing practicality and cost efficiency.
Being accepted into the Shenzhen Pingshan District Medical & Health Data Alliance and having our products listed in the Local Clinical Specialty Medical Devices Catalog affirms XunFengTong’s technological strength and innovation in the rehabilitation field. We view this recognition as both an achievement and a new starting point. We remain committed to our mission—“using innovative technology to enable healthy lives”—and will continue to improve product quality and service standards to contribute to the advancement of the medical device industry.